The following was written by
NH Department of Environmental Services
29 Hazen Drive, PO Box 95, Concord, NH 03302-0095
Environmental, Health and Economic Impacts of Road Salt
Overview
New Hampshire winters demand an effective and affordable means of de-icing roadways. The primary agent used for this purpose is sodium chloride (road salt), which is composed of 40 percent sodium ions (Na+) and 60 percent chloride ions (Cl-). Other components in salt like ferrocyanide, which is used for anti-caking, and impurities like phosphorus and iron, can represent up to 5 percent of the total weight. The sodium, chloride, ferrocyanide and impurities make their way into our environment through the runoff from rain, melting snow and ice, as well as through splash and spray by vehicles and by wind. They find their way onto vegetation and into the soil, groundwater, storm drains, and surface waters causing significant impact to the environment.
Chloride (Cl-) is completely soluble and very mobile. Chloride is toxic to aquatic life and impacts vegetation and wildlife. There is no natural process by which chlorides are broken down, metabolized, taken up, or removed from the environment. In 2008, New Hampshire listed 19 water bodies impaired by chloride; in 2010 that number increased to 40. Trends show that chloride levels continue to rise with the increasing use of road salt.
The transport of sodium (Na+) in the environment is not as prominent as chloride due to ion exchange; however, this exchange can alter the soil chemistry by replacing and releasing nutrients into the groundwater and surface water changing soil structure, and impacting the aquatic environment. Contamination of sodium in drinking water is a concern for individuals restricted to low-sodium diets due to hypertension (high blood pressure). Wildlife is also prone to high sodium levels by ingesting salt or drinking water runoff from snow and ice melt.
Additives to road salt like ferrocyanide, which is used as an anti-caking compound in large salt supplies, can have impacts on both the environment and human health due to cyanide ions being released by certain types of bacteria as well as from exposure to sunlight. The USEPA in 2003 added this compound to its list of toxic pollutants under section 307(a) of the Clean Water Act. Other potential components and impurities of road salt can include calcium, potassium, iron, magnesium, aluminum, lead, phosphorus, manganese, copper, zinc, nickel, chromium, and cadmium.
Water Quality Impacts
Contaminates from road salt enter water resources by infiltration to groundwater, runoff to surface water and through storm drains. The chloride discharged into these waters remains in solution and is not subject to any significant natural removal methods; only dilution can reduce its concentration. During winter and spring and during times of low flow in the summer and fall, chloride levels can exceed 800mg/L, while natural background levels fall within the range of 1-10mg/L. The accumulation and persistence of chloride poses a risk to the water quality and the plants, animals, and humans who depend upon it.
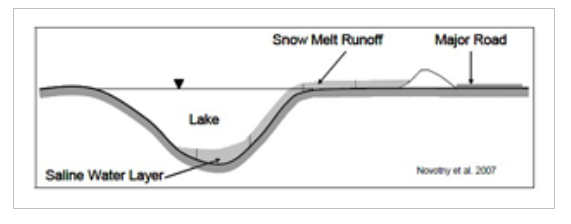
Water contaminated with NaCl creates a higher water density and will settle at the deepest part of the water body where current velocities are low such as in ponds and lakes. This can lead to a chemical stratification which can impede turnover and mixing, preventing the dissolved oxygen within the upper layers of the water from reaching the bottom layers and nutrients within the bottom layers from reaching the top layers. This leads to the bottom layer of the water body becoming void of oxygen and unable to support aquatic life.
The concentration of chloride found in surface water correlates with the proportion of impervious surfaces in the watershed. Chloride cannot be treated or filtered with BMPs, so once the salt is applied, chloride remains in the watershed until it is flushed downstream. Given that groundwater residence time is so much longer, contaminated wells often must be replaced.